You want to enhance quality and performances of your analysis methods through an independent certification? You want to use a brand of quality? You are looking for an experienced and recognized certification body? NF VALIDATION by AFNOR Certification is the answer.
Want your alternative method certified? Contact us.
Reasons to choose AFNOR Certification?
When you choose AFNOR Certification, the European leader in the validation of alternative methods, you choose:
- Over 30 years of expertise in the certification of rapid microbiological test kits;
- A network of experts specializing in the field, independent laboratories and qualified auditors;
- A recognized European brand, well known internationally;
- An independent, competent and reliable body, accredited by Cofrac for NF VALIDATION certification activities (Certification of products and services, accreditation Cofrac No. 5-0030 available at www.cofrac.fr/en/).
In the food microbiology sector, AFNOR Certification is the first European body in granting Certificates according to EN ISO 16140-2 standard (protocol for the validation of alternative (proprietary) methods against a reference method).
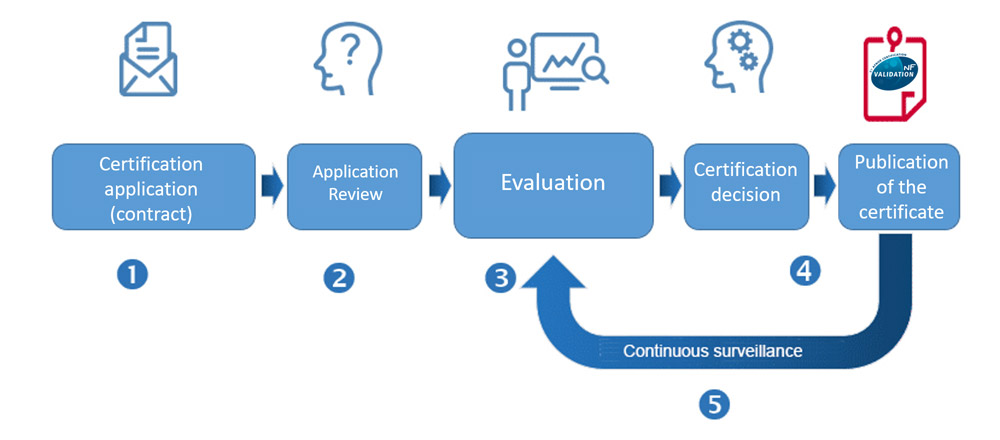
NF VALIDATION certification key steps
Although there may be numerous requirements to be met, NF VALIDATION procedure is simple, in 5 steps:
Contact AFNOR Certification first
We can answer your questions, confirm that your application comes within the scope of application of the NF VALIDATION mark and send you all the practical information you need on the conditions of obtaining certification.
Application for certification
Before starting the NF VALIDATION certification procedure, your alternative method shall be ready for production and your validation project clearly defined (scope, analysis protocols, confirmatory tests…). In addition, the company applying for certification has previously to contact and choose an Expert Laboratory, among ones of the lists of laboratories approved by AFNOR Certification. Then, after receiving your technical application, we initiate the evaluation procedure (date of first submission to Technical Board, estimated audit date).
Fill in the application form and return it to AFNOR Certification!
- Water domain: Application file and List of qualified laboratories
- Agri-food domain: Application file and List of qualified laboratories
An evaluation in 2 stages
The evaluation is based on:
- A validation study: an independent expert laboratory qualified by AFNOR Certification evaluates the performance of the commercial method in accordance with a standardized protocol. The diversity of the samples tested and the experimental conditions applied to the study enable testing in conditions very close to the reality in the field.
- An audit of the production site’s quality system: an auditor qualified by AFNOR Certification verifies that the implemented quality system makes it possible to ensure the production of consistent test kits.
Decision of certification
AFNOR Certification takes the decision to certify an alternative method, taking into consideration the recommendations of the Technical Board on the validation study results, and the conclusions of the auditor on the audit. Once the decision is positive, the NF VALIDATION certificate is granted for a 4-years-period that attests that the analytical performances of the alternative method have been certified. In parallel, a summary report is published, presenting the main results of the validation study. Both documents may be downloaded on this website.
Periodical monitoring
AFNOR Certification carries out regular monitoring in order to check and guarantee users that validated methods comply at all times with the requirements of the NF VALIDATION mark. After a four-years-period, the NF VALIDATION certificate expires. It can be renewed through a renewal process.
Focus on the validation study
Performances characteristics of the alternative method
The method validation study is carried out in 2 phases by an independent Expert Laboratory, according to the current technical protocol:
1st phase: characterization of the alternative method’s performances
The purpose of the first phase (comparative study) is to characterize the method and to assess its performances, by comparison with the reference method or pre-defined performances criteria. This is carried out at the Expert laboratory. The diversity of matrices tested and the experimental conditions applicable to the study (sufficient number of naturally contaminated samples, application of specific stress protocols for artificial contamination…) allow the method to be tested under conditions that are close to the real conditions in the field.
2nd phase: results variability of the alternative method
The purpose of the second phase (or inter-laboratory study) is to determine the variability of the results obtained with the alternative method in several laboratories under defined conditions of reproducibility, and by comparison with the reference method or pre-defined performances criteria.
By combining the results of the 2 phases of the study, it is possible to reach a decision on all of the performance criteria of the method.
Examination by the Technical Board
The Technical Board consists of experts from the concerned domain (food or water microbiology, veterinary drug residues) and represents all stakeholders in methods validation (users, manufacturers, prescribers…). During the different steps of the study (comparative and inter-laboratory), the Technical Board examines study projects and all validation results for each alternative method. For each validation file, two members of the Technical Board are appointed to monitor the validation study closely and give their opinion to the Technical Board.
Each validation study requires three presentations in meeting of the concerned Technical Board:
- Draft method comparison study,
- Results of comparitif study,
- Results of inter-laboratory study.
At each stage, the Expert laboratory in charge of the study presents the application with the manufacturer present. The Technical Board evaluates the application and gives his recommendation on whether to validate the results and the contents of the package insert of the alternative method. At the stage of the results of the inter-laboratory study, a final recommendation on the certification is given.
